Description
Learn how to design and conduct a robust systematic review and meta-analysis for publication
Why conduct and publish Systematic Reviews?
Evidence-based medicine (EBM) is the process of practising medicine based on the best available research evidence, clinical expertise and patient values. Systematic reviews and meta-analyses are fundamental to identify, evaluate, and summarize the findings of all relevant individual studies over a health-related issue. The results of these studies are then pooled and thus the evidence drawn from systematic reviews can be very powerful and valuable, with overall conclusions that are more reliable and accurate than those of individual studies. Moreover, in the advent of COVID-19 and reduced provision for conducting in-lab research, systematic reviews offer the opportunity to conduct remote research that can result in a stand-alone high impact publication. Systematic reviews (with homogeneity) of Randomised Controlled Trials (RCTs) constitute level 1a (highest) in the Oxford Centre for Evidence-Based Medicine hierarchy of evidence:
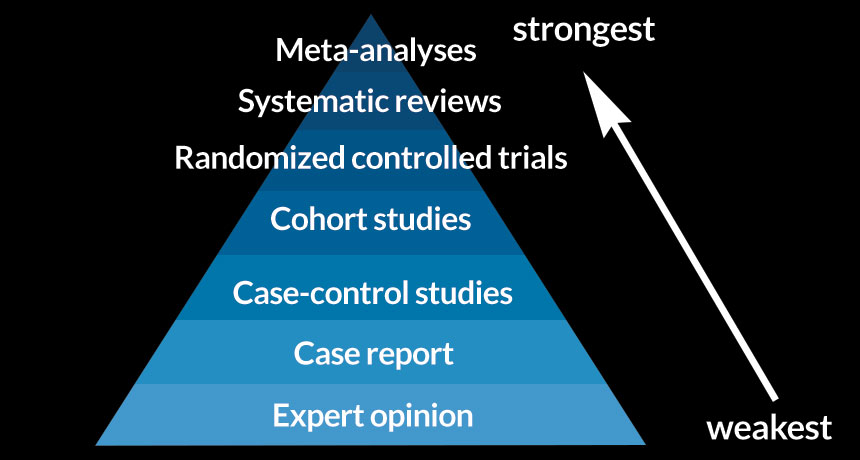
However, there are many challenges in conducting a robust systematic review and meta-analysis:
-
- you may encounter an overwhelming quantity of published studies
- you will need to develop a comprehensive search strategy, and ensure that relevant studies are not missed
- you will need to ensure all relevant databases are searched
- you will need to ensure that appropriate conclusions are drawn in context of the methodological quality and risk of bias in included studies
- you will need to know which tools to use to assess methodological quality and risk of bias AND how
- you will need to register and publish the protocol a priori
- you will need to decide which outcomes to evaluate
- you will need to figure out how to calculate inter-rater reliability
- you will need to determine how to calculate and quantify heterogeneity
- you will need to figure out the difference between random and fixed-effects models and when to use which
- you’ll need to understand how to interpret and perform a meta-analysis
- you’ll need to determine if a meta-analysis is possible or appropriate for your review
- you’ll need to determine whether sub-group analysis is indicated
- And you will have to do all this, undertake revisions, experience rejections while juggling your job, studies, relationships and health, while also trying to enjoy the process and avoid burnout.
This is challenging. Hours can be spent reading books, online articles, watching youtube videos or attending university courses that cost nearly £3,000 GBP (>$4,000 USD), with no guarantee of translating the knowledge to publication.
Instructor story (Dr Ankur Khajuria):
“I became interested in systematic reviews back in 2013, when I conducted my first review. With minimal guidance, I spent countless hours reading books, watching youtube videos, and making a number of mistakes along the way. During my time as a Kellogg Research Scholar at Oxford University, and through experience of taking several reviews from conception all the way through to publication, I began to formulate a system which greatly enhanced the efficiency at each stage of the process. Fast forward 8 years, and I have now published, reviewed and supervised several systematic reviews and meta-analyses. I serve as the Associate Editor of Systematic Reviews Journal, am routinely involved in reviewing papers and I supervise students, having helped multiple students publish their first systematic review.
This course has been designed with all the challenges that I previously faced in mind, and is a programme I wish I had access to when I was starting out. It will take you through the A to Z of systematic reviews and meta-analyses. The aim of the programme is to have you publish your first systematic review. And if you have already published a review before but not felt completely comfortable in your knowledge or skills, the programme will refine your skills and solidify your understanding.”
How to design and conduct a systematic review and meta-analysis for publication in 8 weeks
The programme is a 5-week live online course, with 3 week personal study, where Dr Khajuria will teach you how to design and conduct a robust systematic review and meta-analysis efficiently, without it taking up all of your time and detracting from your clinical training/medical school commitments. The programme and learning objectives are detailed below. All the sessions will be recorded so you can work through the material at your own pace if you prefer. You will have homework assignments in between sessions, to ensure accountability, progress and implementation of the course teaching.
By the end of the programme…
- you will have started your own systematic review with the aim of publication
- you will have developed fundamental understanding of the steps in conducting a systematic review and meta-analysis
- you will have developed a focused research question for yourself using the “Participants Interventions Comparisons Outcomes” (PICO) framework
- you will have develop understanding on how and which outcomes measures to evaluate in your review
- you will have written an effective protocol for registering/publishing a priori
- you will have designed a comprehensive search strategy
- you will have learnt how to efficiently perform article screening
- you will have good understanding of the process used to collect and extract data
- you will have good understanding on which tools to use to assess risk of bias and methodological quality of studies, and how
- you will have developed skills in interpreting and performing meta-analysis using RevMan (Cochrane Collaboration) programme
- you will have developed understanding on how to assess heterogeneity
- subgroup and sensitivity analyses
- you will have access to ~8 hours of video content to review in your own time
- you will receive a certificate for your CV and portfolio
Video content:
Session 1 (1.5 hours):
- Introduction to systematic reviews
- Introduction to meta-analysis
- Four pillar overview – research question, search strategy, quality assessment, data interpretation
- Framing your question using the PICO framework
Session 2 (1.5 hours):
- Protocol development
- Inclusion and exclusion criteria
- Principles of searching/electronic databases and grey literature
- Developing a search strategy
- MeSH/Keywords/Boolean operators
- PRISMA flow diagram
Session 3 (1.5 hours)
- Documenting your search
- Selecting outcomes
- Paper extraction
- Data extraction, collection and management
- Risk of bias assessment
Session 4 (1.5 hours)
- Define sources of bias in the included studies in a systematic review
- Risk of bias assessment
- Methodological quality assessment
- Cochrane Collaboration Risk of Bias tool
- Risk of Bias in Non-randomised Studies-of Interventions (ROBINS-I)
- Measure and quantify heterogeneity
- Introduction to Forest and funnel plots
Session 5 (1.5 hours)
- Data analysis – part 2 – using RevMan
- Systematic review reporting
- Publication bias
Tickets will be non-refundable, but you can have a colleague/friend take your place (please email us highyielduk@gmail.com to schedule the change).






Reviews
There are no reviews yet.